Safety Needles
Needle Gauge (color-coded)
Needle Length
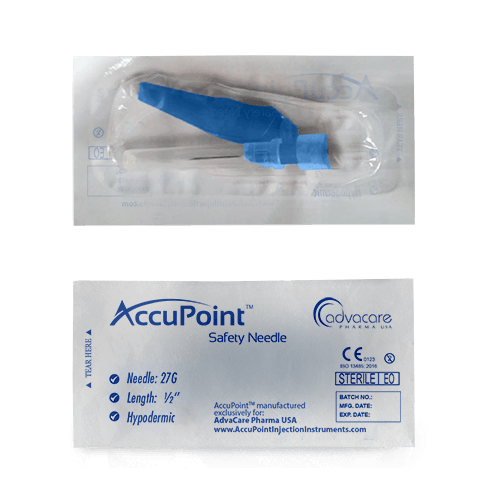
Packaging
What are Safety Needles?
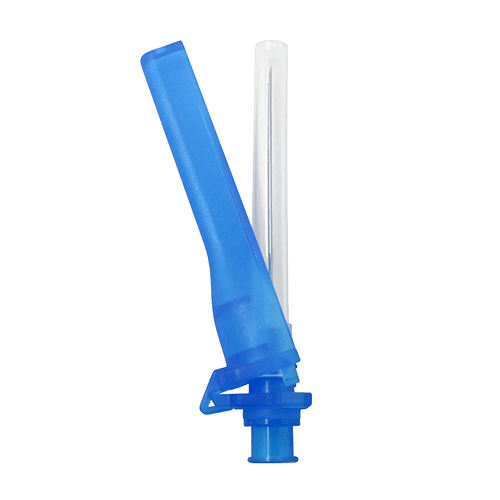
Safety Needles are hypodermic needles that have been designed to minimize the risk of accidental needlestick injuries and prevent reuse. They come in sizes ranging from 16G to 27G and feature a safety mechanism that automatically activates after injection to prevent the needle from being exposed or reused.
Safety needles are also used in situations where injections are self-administered by patients, such as in the case of diabetes management. The use of these needles can help to protect both healthcare workers and patients from the risk of infection or injury.
Our Safety Needles are manufactured in ISO and CE certified facilities in China, India, and the USA, using the latest technologies and best practices to ensure that every product meets our high standards of quality and safety. AdvaCare Pharma is dedicated to improving the lives of patients through innovative medical products.
Product Specifications
Features
Gauges for Safety Needles range from 16G to 27G. Needles with larger gauge numbers have thinner needles and are typically used for less viscous medications, while those with smaller gauge numbers have thicker needles and are used for more viscous medications. The color-coded system is used for easy identification of needle gauge sizes, with larger sizes generally being associated with a darker color. It is important to select the appropriate needle gauge size based on the medication viscosity and the injection site.
Safety needles come with a 45° bevel, which helps to reduce the production of rubber chips during the injection process, thereby decreasing the risk of needlestick injuries. These needles also feature a standard luer hub that is compatible with all standard luer lock/slip syringes. Furthermore, they are designed with a safety cap that makes them suitable for single-use only, preventing the possibility of accidental reuse.
Why are we a trusted Safety Needles manufacturer?
As a leading manufacturer of Safety Needles, AdvaCare Pharma is dedicated to providing reliable and effective medical devices to partners in the healthcare industry. Our AccuPoint™ brand products are manufactured in ISO and CE-certified facilities, and undergo rigorous quality control measures to ensure that our partners receive only the best-quality medical devices.
We supply medical devices to distributors, hospitals, pharmacies, NGOs, and government institutions in over 65 markets worldwide, and our frequent internal and third-party facility inspections ensure that our products meet and exceed the standards required by our partners. All AccuPoint™ medical devices are accompanied by a STED dossier, and we can provide FDA certifications for some product specifications.
At AdvaCare Pharma, we prioritize customer satisfaction and believe in building long-term relationships with our medical distributors. Our commitment to quality and customer service is what sets us apart as a trusted manufacturer and distribution partner.
Uses
How should Safety Needles be used?
To use a safety needle, first remove the protective covering from the needle and attach it to a compatible syringe. Hold the syringe with one hand and use the other hand to prepare the injection site. Administer the medication by inserting the needle into the appropriate location, using the correct technique for the specific injection type.
After administering the medication, activate the safety mechanism by either pushing, pulling, or twisting the needle to cover the needle tip and prevent reuse or accidental needlestick injuries.
How should Safety Needles be disposed of?
After the safety mechanism is activated, dispose of the needle and syringe in a sharps container designated for medical waste. Do not attempt to recap the needle or bend or break it before disposal. Always use a new safety needle for each injection, and do not reuse or share needles. Proper disposal of medical waste is essential to prevent the spread of infection and protect healthcare workers and the general public.
FAQs
How do Safety Needles work?
Safety needles have a built-in safety mechanism that activates after the injection, preventing the needle from being exposed or reused. The mechanism may include a shield or a retractable feature that covers the needle.
What do the different color-coded needle gauges mean?
Safety needles, like regular needles, are color-coded to indicate their gauge size. The smaller the gauge number, the larger the needle diameter, and vice versa. For safety needles, colors may also indicate the safety feature or other specific uses.
How do I choose the appropriate needle length for my injection?
The appropriate needle length depends on the location of the injection site and the depth of the tissue or muscle. A shorter needle may be appropriate for a shallow injection, while a longer needle may be necessary for a deeper injection.
What are the benefits of using Safety Needles?
Safety needles provide an added layer of protection against accidental needlestick injuries and prevent the reuse of needles, reducing the risk of infection and transmission of bloodborne diseases.
How should I store and handle Safety Needles?
Safety needles should be stored in a clean and dry place, away from direct sunlight or heat sources. They should also be disposed of properly after use to prevent accidental needlestick injuries.

You might be interested in...
Why AdvaCare Pharma?
As an industry leader, we are aware of our responsibility to provide affordable and sustainable solutions to improve healthcare worldwide.