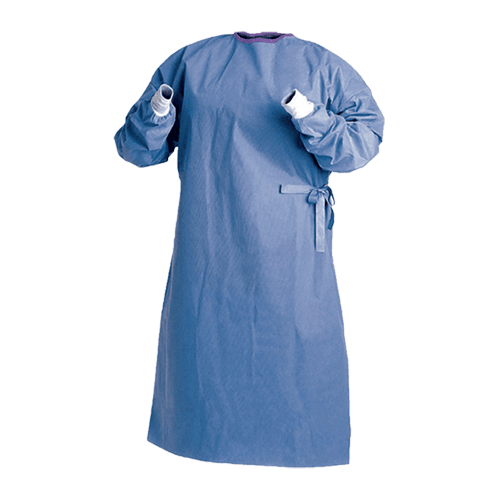
Surgical Gown
Material
Fluid Protection
Collar Closure
Waist Closure
Cuff
Weight
Size
Color
Packaging
What is a Surgical Gown?
A Surgical Gown is a type of personal protective equipment (PPE) worn by healthcare professionals during surgeries or other medical procedures. This garment has been designed to prevent contamination of the patient and surgical equipment. Surgical gowns are produced in a variety of sizes, colors, and styles.
Disposable surgical gowns for doctors and other medical staff are produced in either PP (polypropylene) or SMS (spunbond-meltblown-spunbond). PP gowns offer basic protection and are lightweight, while SMS gowns are more durable and provide better resistance to fluids.
Surgical Gowns are manufactured by AdvaCare Pharma, a reputable pharmaceutical company with expertise in global distribution. This medical disposable product is carefully produced in a CE/ISO-certified facility situated in China, India, and the USA. These facilities are routinely inspected to ensure our products meet health, safety, and environmental standards.
Product Specifications
Material
Fluid Protection
Collar Closure
Waist Closure
Cuff
Polypropylene (PP)
Polypropylene (PP) surgical gowns are made of durable and lightweight polypropylene material, which is waterproof and offers protection against liquids and fluids.
PP is chemical resistant, which adds an additional layer of protection against potentially harmful substances and contamination. It is suitable for settings where protection from fluids and chemicals is crucial.
Spunbond-Meltblown-Spunbond (SMS)
Spunbond-Meltblown-Spunbond (SMS) surgeon gowns are composed of a three-layered material that consists of three layers of spunbond and meltblown fabrics. This unique combination of materials offers excellent strength and durability.
The meltblown layer serves as a protective barrier against bacteria, which enhances the gown's ability to prevent the transmission of infectious agents.
Reinforced
Reinforced surgical gowns are specialized garments for surgeons to wear during hospital surgery or the treatment of patients. These gowns feature reinforced areas, typically in the front and sleeves, that use multiple layers of fabric or additional reinforcement material, typically high-quality non-woven SMS (spunbond-meltblown-spunbond) fabric. This material provides effective fluid resistance, providing a stronger barrier against blood, bodily fluids, and other potential contaminants.
Reinforced surgical gowns meet stringent quality standards and are commonly used in operating rooms, emergency departments, and other sterile environments.

Tie-on
Tie-on collar closures are long fabric straps that can be securely tied at the back of the neck. This design provides a customizable and adjustable fit for healthcare professionals.
Tie-on closures offer versatility in sizing, accommodating various neck sizes comfortably. With this closure style, donning and doffing the isolation gown is both easy and convenient.

Velcro
Velcro collar closures are designed to provide a secure and adjustable fastening system for isolation gowns. The convenient closure style allows for easy and quick adjustment around the neck while also ensuring a comfortable and snug fit.
This closure style is highly adaptable and accommodates various neck sizes while maintaining the gown's integrity during medical procedures.

One Band
One Band waist closures utilize a single band that wraps around the waist, which offers a comfortable fit that can be easily adjusted for the individual wearing the gown.
Healthcare professionals favor this design due to its user-friendly nature. The simplicity and efficiency of the one-band styles allow for the convenient donning and doffing of the gown. It also provides proper coverage and protection during medical procedures.

Four Bands
Four Bands waist closures feature four straps that circle around the waist. This closure style offers improved stability and better ensures that the surgical gown remains securely fastened throughout medical procedures.
The four-band design offers several benefits, including a wider range of adjustability to accommodate various body sizes and enhanced comfort.
Healthcare professionals often prefer this style of waist closure due to its ability to provide a snug and secure fit while maximizing protection and ease of movement.

Knitted
Knitted cuffs are made from a soft and stretchable fabric. They offer an additional layer of protection by acting as a barrier against contaminants.
Knitted cuffs offer a snug and comfortable fit around the wrists. The flexible and gentle nature of the material enhances the wearer's comfort while also maintaining the integrity of the surgical gown.

Elastic
Elastic cuffs are made from a resilient elastic material. They have been designed to provide a secure and comfortable fit around the wrists. By forming a secure seal, this style reduces the possibility of contaminants entering the sleeves.
Elastic cuffs offer a practicality and reliability that is favored by many healthcare professionals.

Why are we a leading Surgical Gown manufacturer?
AdvaCare Pharma is a leading pharmaceutical company that excels in manufacturing Surgical Gowns and other single-use medical devices for surgical procedures. We prioritize the production of high-quality, cost-effective PPE medical supplies. Our highly-skilled team works with our distributors to deliver customized solutions catering to the unique requirements of each market.
Over the past twenty years, we have established ourselves as a trusted and reliable exporter and manufacturer of Surgical Gowns and other medical disposables. Our partnerships include distributors, hospitals, pharmacies, and other many other institutions.
Uses
What is a Surgical Gown used for?
It's used to provide a barrier between the patient and the surgical team member in order to prevent the transmission of microorganisms and maintain a sterile environment.
How is a Surgical Gown worn?
Before donning a surgical gown, it is crucial to wash the hands thoroughly with soap and water or use an alcohol-based hand sanitizer. The arms should be placed through the sleeves of the gown. Any ties or closures should be secured. Ensure the gown covers the chest and back completely. The gown should be inspected for any visible defects, such as tears or holes, as any damaged gown should be discarded.
How should unused Surgical Gowns be stored?
Unused surgical gowns should be stored in a clean and dry area, away from direct sunlight, heat sources, chemicals, and moisture. s. Storing them in their original packaging can help prevent contamination and damage.
How should used Surgical Gowns be disposed of?
Used surgical gowns should be disposed of properly according to healthcare facility protocols and regulations. It is important to handle and dispose of used gowns with caution to minimize the risk of contamination and potential transmission of infectious agents.
FAQs
When should Surgical Gowns be used?
Surgical gowns are commonly worn by surgeons, nurses, and other members of the surgical team in all surgical settings where invasive procedures are performed, including hospitals, clinics, and ambulatory surgical centers.
What are the benefits of PP versus SMS Surgical Gown?
PP surgical gowns are lightweight, breathable, and cost-effective. They provide a good level of protection against fluids and particulate matter, making them suitable for procedures with minimal fluid exposure. SMS surgical gowns are made up of multiple layers and offer a higher level of fluid resistance and better protection against liquid penetration. They are often preferred for procedures with a higher risk of fluid exposure.
What is the difference between a standard Surgical Gown and a reinforced Surgical Gown?
Standard and reinforced surgical gowns differ in their level of barrier protection. Standard gowns are more suitable for procedures with minimal fluid exposure, such as routine surgical procedures. Reinforced gowns have been constructed with additional reinforcement in critical areas like the front chest and sleeves. This type of garment is preferred for procedures with a higher risk of fluid contact, such as during orthopedic surgeries.
You might be interested in...
Why AdvaCare Pharma?
As an industry leader, we are aware of our responsibility to provide affordable and sustainable solutions to improve healthcare worldwide.