- Home›
- Pharmaceuticals›
- Pharmaceutical Tablets›
- Tramadol HCl Tablets
Tramadol HCl Tablets
Dosage
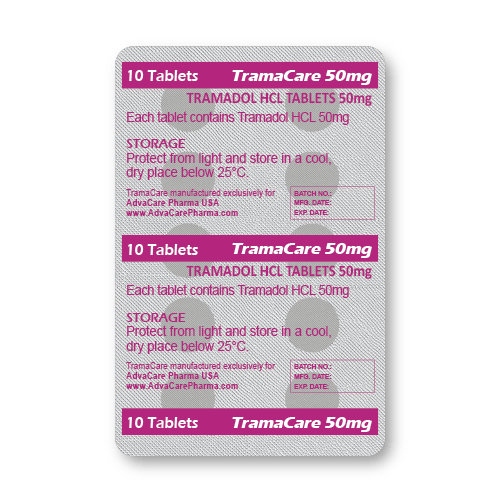
Packaging
What is Tramadol HCl?
Active Ingredients: Tramadol HCl
Tramadol HCl Tablets are used to treat moderate to moderately severe pain in adults. It may be used to relieve symptoms associated with sciatica, neuropathic pain, arthritis, back pain, or postoperative pain. Tramadol may be used for short-term relief or long-term chronic conditions.
Tramadol HCl belongs to the group of medicines called opioid analgesics. The active ingredient works by binding to the mu-opiate receptors of the brain, which change the central nervous system's response to pain.
Tramadol has high tissue affinity and it crosses the blood-brain barrier with peak brain concentrations occurring 10 minutes after oral administration. It also crosses the placental barrier and the umbilical concentrations are ~80% of maternal concentrations. Around 20% of the administered dose is found to bind to plasma proteins. This drug is eliminated primarily through metabolism by the liver and the metabolites are excreted primarily by the kidneys (90% of the excretion is through urine while the remaining 10% is excreted through feces). Around 30% of the dose is excreted in the urine as an unchanged drug, and 60% of the dose is excreted as metabolites.
This medication is produced and exported by AdvaCare Pharma. Tramadol is manufactured in our facilities in China, India, and the USA. Our factories comply with WHO guidelines and standards, and we regularly inspect these facilities to ensure they meet these high standards.
Why choose us as your Tramadol manufacturer?
AdvaCare Pharma is a manufacturer of Tramadol Tablets and other cost-effective and sustainable pharmaceuticals for an ever-changing global market. Tramadol Tablets are a GMP-compliant medicine that is available for distribution.
As a leading Tramadol manufacturer, we work with international partners including pharmaceutical distributors, hospitals, pharmacies and other medical and government organizations. We have established a vested supplier-distributor relationship, ensuring a mutually advantageous outcome during market entry and development.
Uses
What is Tramadol HCl used for?
It is used to manage moderate to severe pain in adults.
How are Tramadol HCl Tablets used?
This medication is intended to be taken orally.
What dose should be taken?
The usual dose for adults is 50mg, taken every 4-6 hours as needed.
The dosage is based on medical condition, response to treatment, age, and weight. Refer to a doctor or pharmacist for guidelines on dosage. Do not exceed what they advise.
Adult Dosing For patients with moderate to moderately severe chronic pain who do not require rapid onset of analgesia, a titration regimen can be used. The daily dose can be increased by 50mg every 3 days, as tolerated, to reach 200mg/day (50mg four times a day). After titration, this drug can be taken in doses of 50mg to 100mg as needed for pain relief every four to six hours. This drug should not exceed 400mg per day.
For patients who need rapid onset of pain relief, tramadol hydrochloride tablets can be administered in doses of 50mg to 100mg as needed every four to six hours, not exceeding 400mg per day.
Pediatric Dosing This medication is not recommended for younger patients than 12 years. Consult with a healthcare professional for more information about the drug.
Renal Dosing For patients whose creatinine clearance is below 30ml/min, it is advised to extend the dosing interval of tramadol hydrochloride tablets to 12 hours, with a maximum daily intake of 200mg. As only 7% of the administered dose is eliminated during hemodialysis, individuals undergoing dialysis can continue with their usual dosage on dialysis days.
Hepatic Dosing For adults with cirrhosis, the suggested dosage is 50mg every 12 hours. When prescribing for elderly patients aged over 65, the dose selection should be made with caution. This drug should be first given in lower doses. For elderly patients aged over 75, the total daily dose should not exceed 300mg.
Who can use Tramadol HCl?
This drug is mainly used to manage severe pain in adults, but special caution should be considered in certain groups of patients.
Pregnant Tramadol belongs to the pregnancy category C and it is shown to be embryotoxic and fetotoxic in animal studies, but was not teratogenic at these dose levels.
There are no available well-controlled studies in pregnant women. Tramadol hydrochloride should be used during pregnancy only if the potential benefit outweighs the potential risk to the fetus.
Labor Tramadol hydrochloride should only be considered for use in pregnant women before or during labor, if the potential benefits outweigh the associated risks. Its safety in pregnancy has not been definitively confirmed. Prolonged use during pregnancy may result in the development of physical dependence and postpartum withdrawal symptoms in the newborn. Tramadol passes the placental barrier and might be a risk for the newborn baby.
Nursing This drug is not recommended for obstetrical preoperative use or for providing post-delivery pain relief to nursing mothers due to a lack of studies assessing its safety in infants and newborns.
Pediatric The safety and effectiveness of tramadol hydrochloride are not well-established in patients under 16 years of age according to some studies and the use of tramadol hydrochloride is not recommended in pediatric patients. This product is not recommended for patients under the age of 12. Before including it to pediatric patients, healthcare providers need to decide if the benefits outweight the risks.
Geriatric In elderly patients, the dose should be selected carefully. This will ensure lower chances of hepatic, renal, or cardiac dysfunctions. For patients over 75 years old, daily doses exceeding 300 mg are not recommended.
Can Tramadol HCl be used concomitantly with other drugs?
Patients should inform their doctors or pharmacists if they are taking any prescribed or unprescribed drugs.
Tramadol should not be used in combination with MAO inhibitors (a class of medications used to treat depression). The pain-relieving effects of tramadol may be reduced or shortened if taken with medications containing carbamazepine (used for epilepsy), pentazocine, nalbuphine, or buprenorphine (painkillers), and ondansetron (used to prevent nausea).
Pain relievers such as morphine and codeine (including cough medicine) and alcohol used concomitantly with tramadol might lead to side effects. This combination may lead to increased drowsiness or fainting. If you notice such symptoms, inform your doctor.
Concurrent use of tramadol and tranquilizers or sleeping pills (e.g., benzodiazepines) can increase the risk of drowsiness, respiratory depression, and coma, and may be life-threatening.
Inform your doctor of all sedative medications you are taking, including tranquilizers, sleeping pills, antidepressants, and other pain relievers like morphine and codeine. It may be helpful to alert friends or relatives about potential symptoms and contact your doctor if experienced.
Medications that may cause seizures, such as certain antidepressants or antipsychotics, can increase the risk of seizures when taken with tramadol.
Coumarin anticoagulants (blood thinners) like warfarin, when taken with tramadol, may affect blood clotting and increase the risk of bleeding.
Other warnings
Patients who are addicted to other pain relievers (opioids) should not take this drug. These patients should receive special medical attention.
Patients who suffer from consciousness disorders should take this drug with caution. Increased pressure in the brain due to head injuries or brain diseases can also lead to side effects.
Inform your doctor if you have difficulties breathing. Patients should also inform their doctors if they have a tendency towards epilepsy.
Inform your doctor if you are taking antidepressants that might interact with tramadol. A so-called serotonin syndrome might occur when tramadol is taken in combination with certain antidepressants.
Seriously ill patients including those with breathing difficulties, patients with excessively low blood pressure (shock), decreased consciousness, serious head injury, or brain diseases that may cause elevated pressure in the skull should take this drug with caution.
Excessive usage of this drug might lead to physical and psychological addiction.
Side Effects
As with all pharmaceuticals, some unwanted effects can occur from the use of Tramadol HCl Tablets.
Common side effects include, but may not be limited to:
- nausea
- drowsiness
- dizziness
- dry mouth
- indigestion
- headache
Seek medical attention if the following develop:
- vomiting
- respiratory depression
- abdominal pain
Sleep-related breathing disorders such as sleep apnoea (breathing pauses during sleep) and sleep-related hypoxemia (low oxygen level in the blood) might occur due to tramadol treatment. The most common symptoms are pauses during sleep, night awakening due to shortness of breath, difficulties maintaining sleep, or excessive drowsiness during the day.
For a comprehensive understanding of all potential side effects, consult a medical professional.
If any symptoms persist or worsen, or you notice any other symptoms, please call your doctor immediately.
Precautions
Do NOT use Tramadol HCl Tablets if:
- You have severe asthma.
- You have liver or kidney problems.
- You are allergic to Tramadol HCl.
- You have recently used alcohol, sedatives, tranquilizers, or narcotics.
- You have recently used an MAO inhibitor, such as isocarboxazid, methylene blue injection, etc.
- You have a history of drug addiction, mental illness or suicide attempts.
Drug interactions may increase the risk for serious side effects or change how the medications work. Consult with a doctor about any current medications before treatment with Tramadol HCl.
Tramadol may not be suitable for people with certain conditions, so it is important to consult with a doctor if you have any health conditions.
This medication is not suitable for children under 12 years.
This drug should be stored in a tightly closed container at room temperature, away from heat, moisture, and direct light. It should be kept out of the reach of children. Unused narcotic medicine should be dropped off at a drug take-back location. If such a location is not available, dispose of it by flushing it down the toilet.
References
An Evaluation of the Efficacy and Tolerability of Oral Tramadol Hydrochloride Tablets for the Treatment of Postsurgical Pain in Children
This is a double-blinded, randomized, multicenter study that examines the analgesic efficacy and tolerability of tramadol in postoperative pediatric patients.
It included 81 pediatric patients aged 7–16 years with ASA physical status I and II who received oral tramadol at doses of approximately 1 or 2mg/kg for postoperative pain management, transitioning from morphine patient-controlled analgesia.
Rescue analgesia included either morphine patient-controlled analgesia or an equivalent oral dose of oxycodone. Pain levels were assessed using the Wong-Baker Faces Pain Rating Scale before and up to 8 hours after tramadol administration. The group receiving 2mg/kg required less rescue analgesia compared to the 1mg/kg group (P = 0.006), a preference noted by their parents. Adverse events, such as vomiting (10%), nausea (9%), pruritus (7%), and rash (4%), were generally mild to moderate and similar between both dosage groups.
The conclusion of this study is that there were no significant changes in hemodynamic parameters, respiratory rate, or oxygen saturation levels between the groups or compared to baseline values for all patients and this makes the drug well-tolerated for younger patients.

You might be interested in...
Why AdvaCare Pharma?
As an industry leader, we are aware of our responsibility to provide affordable and sustainable solutions to improve healthcare worldwide.