- Home›
- Pharmaceuticals›
- Pharmaceutical Tablets›
- Levonorgestrel + Ethinylestradiol Tablets
Levonorgestrel + Ethinylestradiol Tablets
Dosage
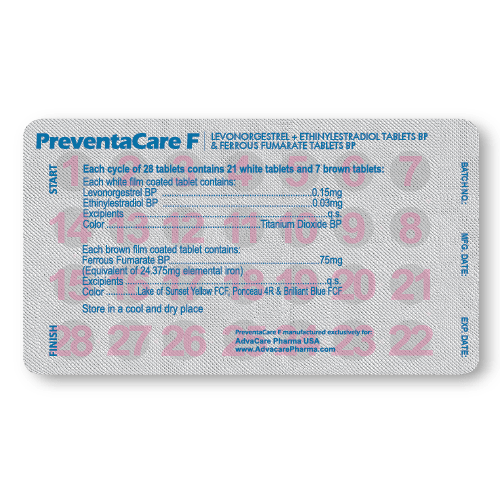
Packaging
What is Levonorgestrel + Ethinylestradiol?
Active Ingredients: Levonorgestrel, Ethinylestradiol, Ferrous Fumarate
Levonorgestrel + Ethinylestradiol Tablets are a combination hormonal birth control medication used to prevent pregnancy. This drug is a reliable reversible method of contraception. It is also used to improve the regularity of the menstrual cycle and reduce pain, blood flow, and premenstrual symptoms.
The combination works to prevent pregnancy by stopping ovulation and altering cervical mucus and the lining of the uterus.
Levonorgestrel is a progestin and ethinylestradiol is an estrogen. The combination works to prevent pregnancy by stopping ovulation and altering cervical mucus and the lining of the uterus.
Ferrous fumarate is an iron supplement that is used to prevent low levels of iron in the blood.
This medication is produced and exported by AdvaCare Pharma. Levonorgestrel + Ethinylestradiol Tablets are manufactured in our facilities in China, India, and the USA. Our factories comply with WHO guidelines and standards, and we regularly inspect these facilities to ensure they meet these high standards.
Why are we a trusted Levonorgestrel + Ethinylestradiol Tablets manufacturer?
We are a Levonorgestrel + Ethinylestradiol manufacturer engaged in the global distribution of 200+ oral solid pharmaceutical products in tablet dosage form.
As an American-owned and operated company, we are devoted to the manufacture and supply of superior quality, yet cost-effective pharmaceuticals to improve access to healthcare solutions worldwide. AdvaCare Pharma manufactures Levonorgestrel + Ethinylestradiol Tablets, and over 500 other pharmaceutical treatments, according to strict GMP regulations as part of our global commitment to bring efficacious medicines to pharmaceutical distributors, hospitals, pharmacies and other medical institutions.
Uses
What is Levonorgestrel + Ethinylestradiol used for?
It is used to prevent pregnancy. It may also be used to regulate the menstrual cycle.
How should Levonorgestrel + Ethinylestradiol Tablets be used?
This medication is intended to be taken orally. These tablets are most effective when taken at the same time every day.
What dose should be taken?
Recommended dosage may vary based on different medical conditions.
Adult Dosing Recommended dosage may vary based on different medical conditions:
- For contraception, the usual dosage is 1 tablet taken at the same time every day. It is recommended to start the pack the first Sunday after menses or day 1 of the menstrual cycle.
- For dysmenorrhea (off-label), the usual dosage is 1 tablet taken at the same time every day. It is recommended to start the pack the first Sunday after menses or day 1 of the menstrual cycle. If pain persists, the inert tablets can be skipped for a continuous cycle.
- For endometriosis (off-label), the usual dosage is 1 tablet taken at the same time every day. It is recommended to start the pack the first Sunday after menses or day 1 of the menstrual cycle. If pain persists, the inert tablets can be skipped for a continuous cycle.
- In the event of an upcoming surgery with VTE risk, this medication should be stopped more than 4 weeks before the surgery and treatment can resume at least 2 weeks after surgery. Therapy can resume 4 weeks after postpartum, or 6 weeks after postpartum for women who are breastfeeding.
Renal Dosing Guidelines have not been defined for patients with renal disease or who are on HD/PD. It is advised to use caution when prescribing to this population.
Hepatic Dosing This medication is not recommended for use in patients with active hepatic disease.
Pediatric Dosing This medicine is only used in postmenarchal females.
Recommended dosage for children may vary based on different medical conditions:
- For contraception in pediatric patients, the usual dosage is 1 tablet taken at the same time every day. It is recommended to start the pack the first Sunday after menses or day 1 of the menstrual cycle.
- For dysmenorrhea (off-label) in pediatric patients, the usual dosage is 1 tablet taken at the same time every day. It is recommended to start the pack the first Sunday after menses or day 1 of the menstrual cycle. If pain persists, the inert tablets can be skipped for a continuous cycle.
- For endometriosis (off-label) in pediatric patients, the usual dosage is 1 tablet taken at the same time every day. It is recommended to start the pack the first Sunday after menses or day 1 of the menstrual cycle. If pain persists, the inert tablets can be skipped for a continuous cycle.
The dosage is based on medical condition, response to treatment, age, and weight. Refer to a doctor or pharmacist for guidelines on dosage. Do not exceed what they advise.
What should I do if I miss a dose?
Please review the specific patient information for this medication or call a doctor for any clarification. Missing a dose increases the risk of pregnancy. If you miss:
- 1 active pill: take this pill as soon as you can and take the next dose at the regular time. This may mean 2 pills are taken within one day.
- 2 active pills within week 1 or 2: take two pills as soon as possible and 2 pills the following day. It is important to use a secondary form of birth control for 7 days afterward.
- 2 active pills within week 3 (or) 3+ active pills in weeks 1, 2 and 3: Start a new pack of this medication. It is important to use a secondary form of birth control for 7 days afterward.
Who can use Levonorgestrel + Ethinylestradiol?
Levonorgestrel + Ethinylestradiol Tablets can be given to adults and children, but caution is advised for specific groups of patients.
Pregnant As the primary indication of Levonorgestrel + Ethinylestradiol is contraception, this medication is contraindicated for use by pregnant women. Based on human data, there is no known risk of teratogenicity if it is accidentally taken during early pregnancy.
Breastfeeding It is recommended to avoid using this medication for at least 6 weeks postpartum. If necessary, use non-hormonal or progestin-only options. After 6 weeks, the benefits should be weighed against the risks of this medication. Though no human data is available to appropriately evaluate risks for the nursing infant, there is a risk of possible gynecomastia based on higher estrogen doses. This drug is associated with decreased milk production.
Children Levonorgestrel + Ethinylestradiol can be used in female patients who are postmenarchal. It is not indicated for males or females who have not started their menstrual cycle. The safety and efficacy are expected to be the same for both postpubertal adolescents over 16 years old and those who are younger than 16.
Geriatric This medication is not indicated for women who are past menopause.
Other warnings
There is an increased risk of venous thromboembolism (VTE) after delivery when the patient is also taking combined hormonal contraceptives. After 21 days, this risk declines rapidly. After 42 days, it returns to normal.
After vaginal birth, patients should wait at least 3 weeks before beginning oral contraception. After cesarean section, patients should wait at least 6 weeks.
For women with elevated risk for VTE in addition to postpartum, this medication is not recommended.
Smoking increases the risk of severe heart problems from the use of this medication. This risk increases with age beginning at age 35, and further increases with heavy smoking (greater than 15 cigarettes per day). Due to these increased risks, this medication is not recommended for patients who smoke cigarettes.
Due to the risk of ALT elevations, this medicine should not be used for patients who are treating hepatitis C with drugs such as ombitasvir, paritaprevir, or ritonavir, with or without dasabuvir.
This medication is contraindicated for the following conditions:
- current or past breast cancer or other estrogen- or progestin-sensitive cancer
- estrogen-dependent neoplasia
- liver tumors, benign or malignant
- liver disease
- acute viral hepatitis
- severe cirrhosis
- abnormal vaginal or uterine bleeding
- history of jaundice due to hormonal birth control or pregnancy
- uncontrolled hypertension
- hypertension with vascular disease
- diabetes mellitus and over 35 years old
- diabetes mellitus with hypertension, vascular disease, or other end-organ damage
- diabetes mellitus for over 20 years
- inherited or acquired hyper-coagulopathies
- high risk of arterial or venous thrombotic or /thromboembolic disease
It is advised to use caution for patients with a family history of breast cancer, DVT or PE, current or past depression, endometriosis, diabetes mellitus, hypertension, renal or hepatic impairment, bone metabolic diseases, or systemic lupus erythematosus. Care should also be used for patients with conditions exacerbated by fluid retention.
Side Effects
As with all pharmaceutical medicines, some unwanted effects can occur from the use of Levonorgestrel + Ethinyl Estradiol.
Common side effects include, but may not be limited to:
- nausea
- vomiting
- diarrhea
- heavier or lighter menstrual periods
- spotting between menstrual periods
Serious side effects may include: • severe lower abdominal pain
For a comprehensive understanding of all potential side effects, consult a medical professional.
If any symptoms persist or worsen, or you notice any other symptoms, please call your doctor immediately.
Contraception with levonorgestrel, 0.15mg, and ethinyl estradiol, 0.03mg. Clinical studies in Latin America
This study examines the effectiveness and safety of an ultra-low-dose oral contraceptive containing 0.15mg of levonorgestrel and 0.03mg of ethinyl estradiol. The research followed 1,206 sexually active, fertile women in their reproductive years over 9,736 cycles.
The results showed that only one out of every eight pregnancies was attributed to the failure of the medication, resulting in a corrected Pearl index of 0.13. The contraceptive demonstrated excellent regulation of menstrual cycles with a low rate of intermenstrual bleeding. Discontinuation of the medication due to medical reasons occurred in only 5% of the patients. Except for a mild case of thrombophlebitis, there were no significant side effects observed.
The findings from this study show that this combination oral contraceptive offers effective contraception with a minimal incidence of side effects.
Precautions
Do NOT use Levonorgestrel + Ethinylestradiol Tablets if:
- You are allergic to any of the ingredients.
- You have a history of heart attack or stroke.
- You have a history of blood clots in the legs (thrombophlebitis), lungs (pulmonary embolism), or eyes.
- You have chest pain (angina pectoris).
- You have breast cancer or cancer of the lining of the uterus, cervix, vagina, liver cancer, or certain hormonally sensitive cancers.
- You have unexplained vaginal bleeding.
- You have diabetes.
- You are pregnant or may be pregnant.
Before treatment, consult your doctor regarding any medications you are taking to address potential drug interactions.
This medication may not be suitable for people with certain conditions, so it is important to consult with a doctor if you have any health conditions.

You might be interested in...
Why AdvaCare Pharma?
As an industry leader, we are aware of our responsibility to provide affordable and sustainable solutions to improve healthcare worldwide.