- Home›
- Medical Devices›
- Injection Instruments›
- Blood Transfusion Sets›
- Hemodialysis Catheter Kit
Hemodialysis Catheter Kit
Extension Line Type
Lumen Type
Lumen Size
Catheter Length
Kit Components
Packaging
What is a Hemodialysis Catheter Kit?
A Hemodialysis Catheter Kit is a set of medical devices used to achieve temporary vascular access during hemodialysis procedures. The kit is available in straight or pre-curved extension lines. It also has single, double, or triple lumen types and catheter lengths ranging from 6.5 Fr to 12 Fr.
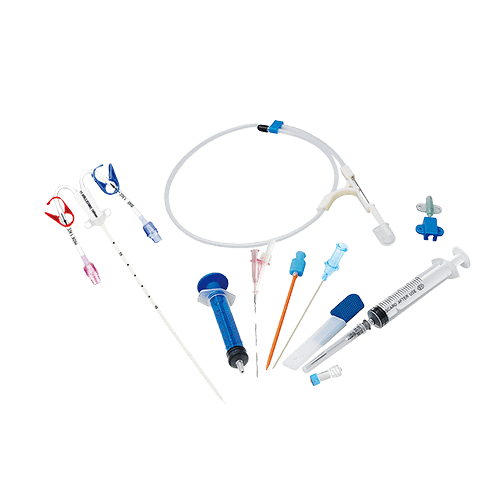
The components of a hemodialysis catheter kit typically include a catheter, introducer needle, guidewire, dilator, injection cap, clamp, and syringe. The catheter itself is usually made of polyurethane material, which is biocompatible, radiopaque, and kink-resistant. The kit is designed for ease of use and reduces the risk of infection or other complications associated with hemodialysis catheterization.
AdvaCare Pharma's Hemodialysis Catheter Kit is manufactured in ISO and CE certified facilities in China, India, and the USA. These facilities undergo regular inspections to maintain compliance with the highest safety, quality, and environmental standards.
Product Specifications
Extension Line Type
Lumen Type
Kit Components
Straight Extension Line
Straight extension line hemodialysis catheter kit is selected based on medical criteria such as the insertion site, which could be the jugular, subclavian, or femoral veins, and the patient's size. It provides a direct path from the catheter to the dialysis machine, allowing blood to flow easily. The single line is used for a single lumen catheter.
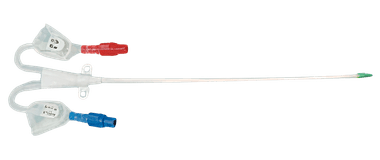
Pre-curved Extension Line
Pre-curved Extension Line hemodialysis catheter kit is specifically designed to be used for jugular insertions and to provide fixation of the catheter and a barrier against infection. The selection of the pre-curved line is based on medical criteria that considers the patient's size and the insertion site of the catheter, such as the jugular, subclavian, or femoral veins.
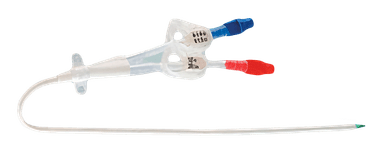
Single Lumen
Single Lumen hemodialysis catheter has one central hole through which the blood is withdrawn and returned to the patient during the hemodialysis procedure. It is suitable for patients who require temporary vascular access for hemodialysis, and its size typically ranges from 6.5 Fr to 12 Fr. The single lumen catheter is inserted into the jugular, subclavian, or femoral vein, depending on medical criteria and patient size.

Double Lumen
Double Lumen hemodialysis catheter has two large bore lumens that enable blood to flow out of the patient and into the dialysis machine before returning it to the patient. This configuration enables the machine to create a complete circuit for the removal and return of blood during treatment. With a maximized internal diameter, double lumen catheters provide consistent and equal flow rates, making them ideal for patients requiring high blood flow rates.

Triple Lumen
Triple Lumen hemodialysis catheter has three lumens, two for blood access and return, and one independent port for the administration of medication or fluids between treatments. This design allows for more versatile usage, especially in critical care settings where multiple interventions may be required. Triple lumen dialysis catheters are typically used for patients who require frequent blood draws or medication administration during dialysis treatments.

Polyurethane Catheter (PU catheter)
Polyurethane Catheter (PU catheter) is a flexible, biocompatible catheter made from polyurethane material that provides excellent durability and biostability. It is designed to resist kinking and collapse, ensuring consistent and effective blood flow while performing dialysis treatments. The PU catheter is also radiopaque, which allows for easy visualization during placement and monitoring.
Vessel Dilator
Vessel Dilator is a medical device used in the placement of hemodialysis catheters. It is a tapered plastic rod that is used to dilate the vein and make it easier to thread the catheter into the desired location. The dilator is gently inserted into the vein and then removed, leaving the larger opening for the catheter to be inserted.
Guidewire
Guidewire is a thin, flexible wire that is used to guide the catheter through the veins and into the correct position. It is typically made of stainless steel or nitinol, and comes in various lengths and diameters to accommodate different patient sizes and insertion sites. The tip of the guidewire is designed to navigate through the veins with minimal resistance and trauma.
Clamp
Clamp is a device used in hemodialysis catheter sets to temporarily interrupt the flow of fluid or blood. It is usually made of plastic or metal and can be opened or closed by squeezing or releasing a lever. The clamp can be used to prevent backflow of blood or to facilitate catheter connection and disconnection.
Disposable Syringe (5ml Luer Slip)
Disposable Syringe (5ml Luer Slip) is a sterile, single-use syringe designed for the safe and accurate delivery of medication or fluid. Its luer slip tip securely attaches to the catheter or extension line and its clear barrel allows for easy and precise measurement of the desired volume. The syringe is designed to reduce the risk of contamination and cross-infection during dialysis.
Injection Cap
Injection Cap is a small plastic cap that is used to cover the end of the catheter when it is not in use. It is designed to provide a sterile barrier and prevent contamination. The injection cap is also used to allow medication or other fluids to be administered directly into the catheter.
Introducer Needle (18G)
Introducer Needle (18G) is a sharp, hollow needle used to puncture the skin and underlying tissue to access the blood vessels. It is part of the hemodialysis catheter insertion set and is used to create an entry point for the guidewire and catheter. The needle is typically removed once the guidewire is inserted, leaving the guidewire in place for the catheter to be advanced over.
Scalpel (11)
Scalpel (#11) is a surgical instrument that is included in dialysis catheter kits to facilitate catheter insertion. It is a small, sharp knife with a pointed blade that is used to make small incisions in the skin. The scalpel is designed for precision cutting and is commonly used in medical procedures that require a high degree of accuracy.
Why are we a quality Hemodialysis Catheter Kit manufacturer?
AdvaCare Pharma is a leading global supplier and manufacturer of Hemodialysis Catheter Kits that are ISO and CE-certified and comply with global healthcare standards through both internal and third-party assessments.
With a strong supplier-distributor partnership and distribution networks in 65 markets, we offer innovative solutions and improved access to superior healthcare products. Medical professionals trust AdvaCare Pharma as a supplier of medical devices because we manufacture under strict quality guidelines, have obtained various international certifications, and offer cost-effective solutions without compromising quality and safety.
Uses
How is a Hemodialysis Catheter Kit used?
Hemodialysis Catheter Kits are designed for professional use to obtain vascular access for dialysis treatment. Before starting, professionals must check the kit is integrity and sterility.
The procedure requires precise insertion of the catheter into the targeted vein, followed by secure anchoring. Throughout this process, maintaining exemplary hand hygiene is paramount to minimize infection risks.
The kit typically includes a catheter, introducer needle, guidewire, dilator, injection cap, clamp, and syringe. Made from biocompatible polyurethane, the catheter is designed for durability and to prevent kinking, establishing uninterrupted blood flow and reducing complications during dialysis.
How should a Hemodialysis Catheter Kit be safely removed and disposed of?
After use, the Hemodialysis Catheter Kit must be removed and disposed of with care to uphold safety and comply with health regulations. Components, especially sharps like needles and syringes, should be placed in designated containers to prevent injuries.
The catheter's removal should be gentle to avoid displacing it unexpectedly. Cleansing and disinfecting the site post-removal is imperative.
Disposal of the kit and any non-used items must follow the facility's protocols for hazardous waste, providing a safe environment for both patients and healthcare staff.
How do medical professionals utilize the Hemodialysis Catheter Kit?
Healthcare providers, specifically trained in dialysis procedures, utilize the Hemodialysis Catheter Kit in various settings, including hospitals and dialysis centers.
Their skill is all-important for the accurate placement and fixation of the catheter, adhering to strict hygiene standards to lower contamination risks.
The comprehensive design of the kit, with its assorted components, supports a smooth dialysis session, emphasizing patient safety and procedural efficacy.
FAQs
What are the parts of a Hemodialysis Catheter Kit and how do they interact?
The hemodialysis catheter kit typically includes a catheter, guidewire, dilator, introducer needle, clamp, syringe, and injection cap. These parts work together to create temporary vascular access for hemodialysis. The introducer needle is used to puncture the vein and create a pathway with the guidewire and the dilator for the catheter to be inserted, while the clamp and injection cap are used to regulate blood flow and prevent infection.
What is the difference between single, double, and triple lumen?
Single lumen kits have one catheter and are used for hemodialysis only, while double lumen kits have one catheter with two channels (lumens), one for blood withdrawal and the other for blood return during the hemodialysis procedure. Triple lumen kits have an additional catheter for infusion or medications during the hemodialysis procedure.
What are the different catheter diameter available for Hemodialysis Catheter Kit?
Catheter diameter range from 6.5 Fr to 12 Fr, with the most common diameter being 8 Fr. The appropriate diameter for a patient depends on their body size, the location of the catheter insertion site, and the expected duration of use.
What is the difference between straight and pre-curved extension line types?
Straight extension lines are ideal for patients who require a high flow rate during hemodialysis, while pre-curved extension lines are ideal for patients with difficult vascular access or those who require long-term catheterization. The pre-curved extension lines are designed to reduce the risk of the catheter kinking or becoming occluded.
How should Hemodialysis Catheter Kits be stored and handled?
Kits should be stored in a clean, dry place at room temperature. They should be inspected for any signs of damage or contamination before use, and any damaged or expired components should be discarded. The dialysis catheter set should be handled with care to prevent damage to any of the components, and the insertion site should be cleaned and disinfected according to standard medical practices.
Do you offer technical support for your Hemodialysis Catheter Kits?
Although most of our Class I and Class II medical devices are user-friendly and require minimal training, we offer extensive documentation and auxiliary technical resources. Our team is also on standby to provide support to our distributors whenever needed.
Is it challenging to import Hemodialysis Catheter Kits and other Class II medical devices for distribution?
Importing Class II medical devices may involve more regulatory oversight than Class I devices; however, with proper documentation and compliance, they can still be imported for distribution. Our Regulatory Affairs Department provides expert assistance to ensure efficient registration in the target country.
What steps should I take to get pricing information for your Hemodialysis Catheter Kits?
Pricing information for our medical devices is available upon request. Please contact our International Sales Department for a quotation and to discuss distribution opportunities.
References
Haemodialysis catheters - a review of design and function
This study focuses on the design and function of haemodialysis catheters, exploring factors such as materials, surface coatings, tip geometry, side holes, and lumen cross-sectional shape. The study emphasizes the need for thoughtful catheter insertion and maintenance practices over mere device selection based on theoretical design principles.

You might be interested in...
Why AdvaCare Pharma?
As an industry leader, we are aware of our responsibility to provide affordable and sustainable solutions to improve healthcare worldwide.